

"Inosilicates": Chain silicates
Si:O ratio is 4 : 11
Examples of Amphiboles:
Also click here for a triangular compositional diagram of the amphiboles
- water (OH-) is common in the holes at lower temperatures but
can be driven out of structure with heating
- when the water is driven out, the structure may convert to a
pyroxene
http://classes.colgate.edu/rapril/geol201/
Double chain silicates = "Amphiboles" - Si4O11
Tetrahedra alternate sharing 2, then 3, then 2, then 3... oxygens with neighboring tetrahedra:
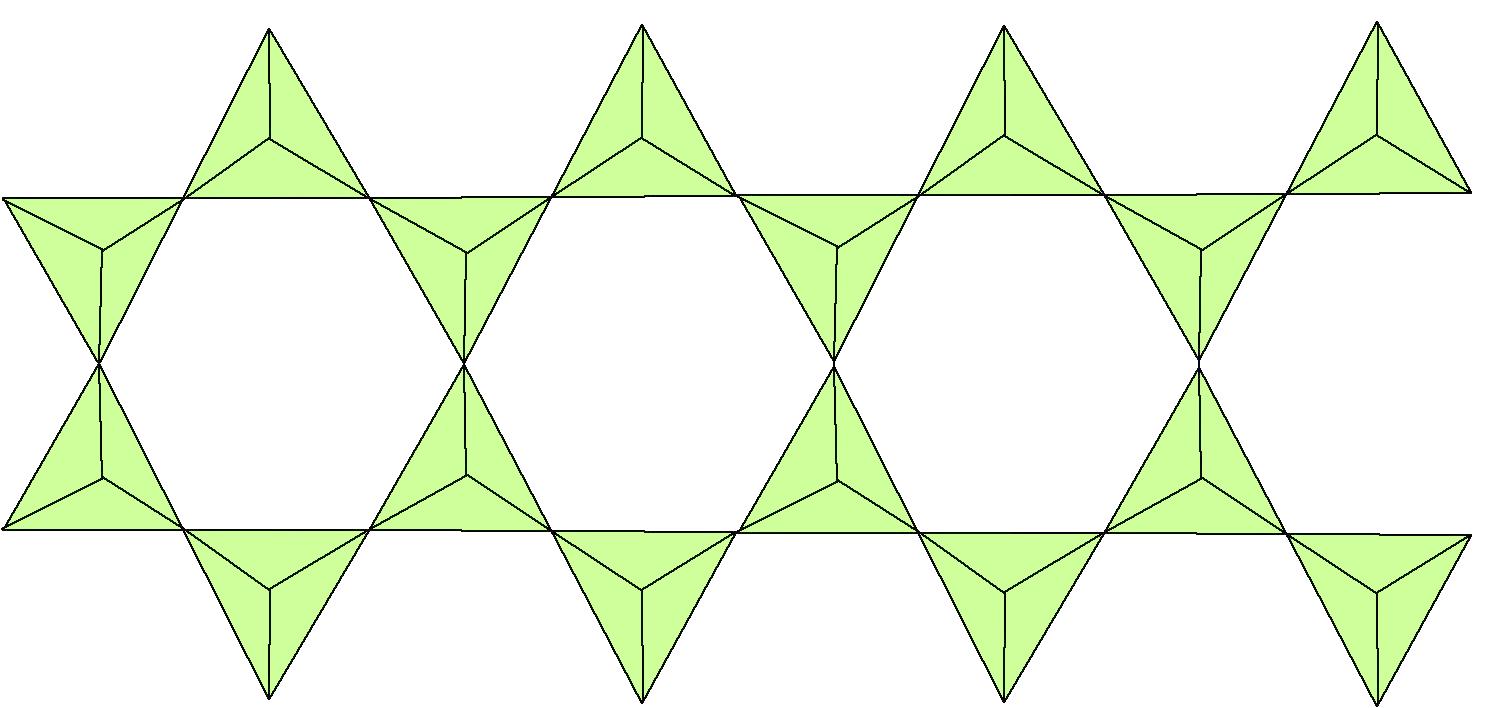
Hornblende (Ca,Na)2-3(Mg,Al,Fe)5Si6(Si,Al)2O22(OH)2
Tremolite Ca2Mg5Si8O22(OH)2
Actinolite Ca2(Mg,Fe)5Si6(Si,Al)2O22(OH)2
- this hole is filled by large anions like OH-, Cl-, F-
Back to Main Silicate Page
Questions to:
rapril@mail.colgate.edu
Copyright 1997 © Colgate University.