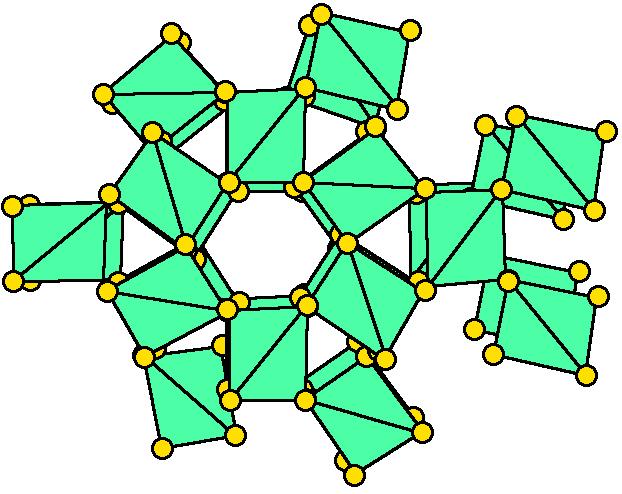
Tectosilicates
Tectosilicates
"Tectosilicates": Framework silicates - SiO2
Examples of Tectosilicates:
Quartz - SiO2
Orthoclase KAlSi3O8
Albite NaAlSi3O8
Labradorite (Ca,Na)(Al,Si)4O8
Anorthite CaAl2Si2O8
SiO2 Group
2 Polymorphs of Quartz:
Low Quartz (low pressure/low temp. conditions)- Hex/rhomb system
High Quartz (low pressure/high temp. conditions) - hex/hex system
Tridymite
High Tridymite - hex.hex system
Low Tridymite - orthorhombic system
Cristobalite
High Cristobalite - isometric system
Low Cristobalite - tetragonal system
Coesite
Stishovite
|
Stability Fields for the SiO2 Group
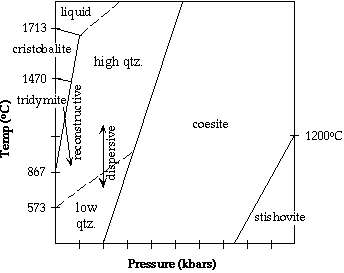
|
One method that the SiO2 structure changes from one polymorph to another is
by:
Reconstructive Transformation
- this involves the breaking and reforming of bonds
- the process is slow (may take a million years) and irreversible
Feldspar Group - (SiO2)4 = Si4O8
- Al+3 substitutes for 1 - 2 Si+4
to balance the charge difference and allow the addition of other cations
For example:
Click here for a triangular compositional diagram of the feldspars
|
http://classes.colgate.edu/rapril/geol201/
Questions to:
rapril@mail.colgate.edu
Copyright 1997 © Colgate University.
|
|