|
Different stacking arrangements of tetrahedral sheets and octahedral sheets, along with
the type of cation that occupies the octahedral site, allow for the variety
of phyllosicilicates that occur in nature.
Stacking the Sheets
These stacking patterns include:
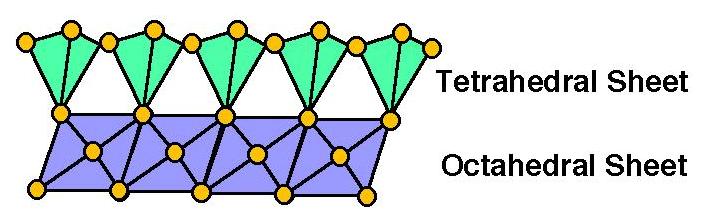
1 : 1 Phyllosilicates
have a "T-O" stacking pattern - 1 Tetrahedral Sheet for every 1 Octahedral Sheet
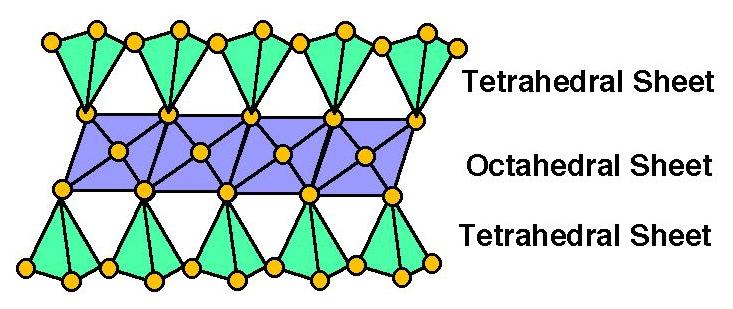
2 : 1 Phyllosilicates
have a "T-O-T" stacking pattern - 2 Tetrahedral Sheets for every 1 Octahedral Sheet
Octahedral Sheet Varieties
Octahedral varieties include:
Dioctahedral
- cations in a dioctahedral sheet are TRIVALENT (+3) (usually Al+3)
- the -6 charge from the anions is satisfied by only two trivalent cations
so one out of every three cation sites is vacant
- each O or OH is bonded to TWO cations
Trioctahedral
- cations in a trioctahedral sheet are DIVALENT (+2) (usually Mg or Fe+2)
- the -6 charge from the anions is satisfied by three divalent cations
so all cation sites are filled
- each O or OH is bonded to THREE cations
Common Phyllosilicates:
1 : 1 "T-O" Phyllosilicates: (Si2O5)
Dioctahedral: Kaolinite Al2Si2O5(OH)4
Trioctahedral: Serpentine (Mg,Fe)3Si2O5(OH)4
2 : 1 "T-O-T" Phyllosilicates: (Si4O10)
Dioctahedral: Pyrophyllite Al2Si4O10(OH)2
Trioctahedral: Talc (Mg,Fe)3Si4O10(OH)2
2 : 1 "T-O-T" + Interlayer Cation Phyllosilicates: (Si4O10)
The Micas:
Dioctahedral: Muscovite KAl2(Si3Al1)O10(OH)2
Trioctahedral: Biotite K(Mg,Fe)3(Si3Al1)O10(OH)2
2 : 1 "T-O-T" + Interlayer Octahedral Sheet Phyllosilicates: (Si4O10)
Chlorite - both Dioctahedral & Trioctahedral Varieties:
Di-Dioctahedral: [Al(OH)3]Al2(Si4)O10(OH)2
Di-Trioctahedral: [Al(OH)3](Mg,Fe)3(Si4)O10(OH)2
Tri-Dioctahedral: [(Mg,Fe)(OH)3]Al2(Si4)O10(OH)2
Tri-Trioctahedral: [(Mg,Fe)(OH)3](Mg,Fe)3(Si4)O10(OH)2
|