

"Cyclosilicates": ring silicates - SiO3
6-member rings are the most common
3, 4 or 6 tetrahedra linked - each tetrahedron shares a total of 2 oxygens
with two different neighboring tetrahedra, building ring structures
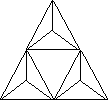 3-member ring - Si3O9 |
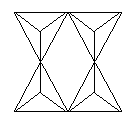 4-member ring - Si4O12 |
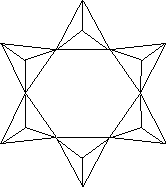 6-member ring - Si6O18 e.g., beryl, tourmaline |
Examples of Cyclosilicates:
Tourmaline (Na,Ca)(Li,Mg,Al)(Al,Fe,Mn)6(BO3)3(Si6O18)(OH)4
| Back to Main Silicate Page |
|
http://classes.colgate.edu/rapril/geol201/ |