

Unit Cell: The smallest unit of a crystal that still retains all the
physical, chemical and crystallographic properties of the mineral.
The 4 pure symmetry operations (which are operations that leave one point in the crystal unmoved) include:
|
Rotation |
|
|
|
|
|
|
Notation |
|
|
|
|
|
| Symbol |
|
|
|||
|
Needed to Repeat Image |
|
|
|
|
|
|
|
|
|
|
|
| |
|
| |
|
|
| |
|
|
(x=fold of rotation) |
|
|
|
|
|
|
|
|
|
|
Crystal systems:
| System Name | Shared Symmetry | Axial Lengths | Angles |
| Triclinic | i or none | a ≠ b ≠ c | α ≠ β ≠ γ no angle = 90° |
| Monoclinic | 1A2 and/or 1m | a ≠ b ≠ c | β ≠ α = γ = 90° |
| Orthorhombic | 3A2 and/or 1A22m | a ≠ b ≠ c | α = β = γ = 90° |
| Tetragonal | 1A4 | a1 = a2 ≠ c | α = β = γ = 90° |
| Hexagonal |
hex/hex:1A6 |
a1 = a2 = a3 ≠ c |
β1 = β2 = β3 = 90° γ1 = γ2 = γ3 = 120° |
|
hex/rhomb:1A3 |
a1 = a2 = a3 = c | ||
| Isometric | 4A3 | a = b = c | α = β = γ = 90° |
Oblique 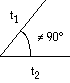 t1 < t2 t1 < t2
|
Rectangular 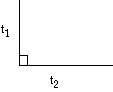 t1 < t2 t1 < t2
|
Square 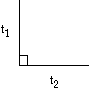 t1 = t2 t1 = t2
|
Rhombic 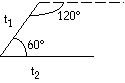 t1 = t2 t1 = t2
|
|
Centered Rectangular of two adjacent rectangles. i.e., |
|
Formed by extending planar nets into 3rd dimension. Atom positions within Bravais Lattices can occur as follows:
Zone: Faces of a crystal that intercept such that their edges run parallel to each other make up a "zone."
Form: Faces that share the same relationship to the symmetry that is present in a crystal are grouped together as a "form."
|
http://classes.colgate.edu/rapril/geol201/ |