

-white light - no visible wavelengths absorbed - see clear or white crystal
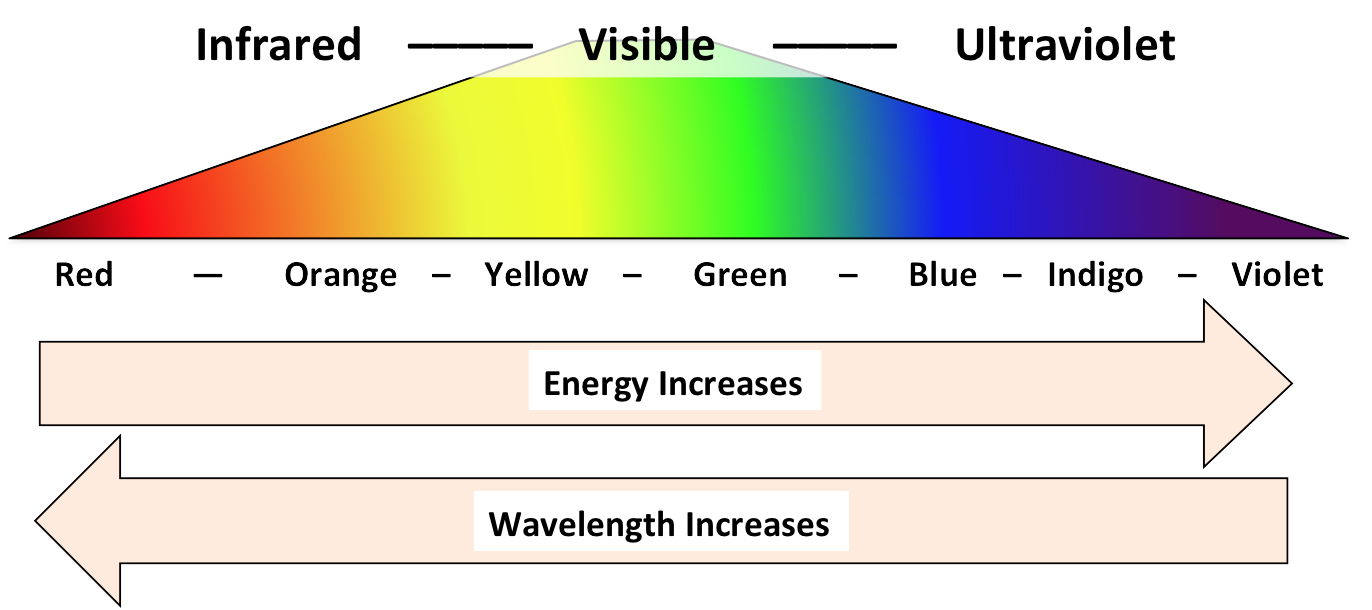
Color-Causing Mechanisms
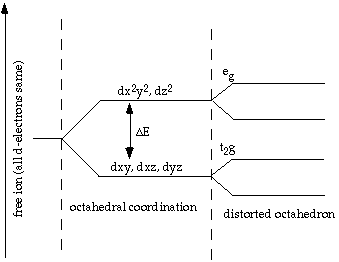
For Fe2+ there are 6 d electrons spread among 5 d orbitals
Absorption of a photon would simply cause the second electron in the pair to pair up with one of the unpaired electrons. The result would still be 4 unpaired and 1 paired.
i.e., before transition: 4 UNPAIRED, 1 PAIRED
Energy needed for an electron to make the transition between d orbitals is more when the number of paired electrons within the 5 orbitals is different after the transition. This is called a "Spin Forbidden" Transition.
For Fe3+ there are 5 d electrons spread among 5 d orbitals
Absorption of a photon would cause one of these unpaired electrons to jump up and pair with one of the other unpaired electrons. The result would be 3 UNPAIRED and 1 PAIRED.
i.e., before transition: 5 UNPAIRED, 0 PAIRED
Effect of Bond Strength on Color
Same rules as above for Spin Allowed and Spin Forbidden Transitions (see "Effect of Valence on Color") except that electrons are transferring between cations not just changing orbitals within one cation.
|
Cation |
# Unpaired |
# Paired | BEFORE TRANSITION |
Ti4+ there are 0 d electrons spread among 5 d orbitals |
0 |
0 |
Fe2+ there are 6 d electrons spread among 5 d orbitals |
5 |
1 |
AFTER TRANSITION |
Ti3+ there is 1 d electron spread among 5 d orbitals |
1 |
0 |
Fe3+ there are 5 d electrons spread among 5 d orbitals |
5 |
0 |
Therefore this is a Spin-Forbidden Transition => higher energy wavelengths are absorbed
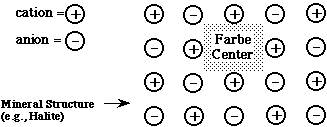
In the instance of a mineral like halite, with strong ionic bonds, the energy gaps (difference in energy between electron sites) is too great for photons in the visible range to be absorbed.
Farbe Centers provide new lower energy "sublevels" into which electrons can drop. The energy gaps between these sublevels and the main levels are within the visible light range.
|
http://classes.colgate.edu/rapril/geol201/ |