 Atomic Substitution/Isomorphism
Atomic Substitution/Isomorphism
According to Goldschmidt's Rules atomic substitution is controlled
by:
- the size (i.e., radii) of the ions
- free substitution can occur if size difference is less than ~15%
- limited substitution can occur if size difference is 15 - 30%
- little to no substitution can occur if size difference is
greater than 30%
- the charge of the ions --> cannot differ by more than 1
Isomorphs: minerals with different chemical compositions; same crystal structure
(belong to same crystal class)
Polymorphs: minerals with same chemical composition; different crystal structures
When the chemical composition of a mineral varies because of atomic
substitution, the mineral is said to exhibit "Solid Solution"
Solid Solution is defined as "a mineral structure
in which specific atomic site(s) are occupied in variable proportions by two or
more different elements." (Klein & Hurlbut, p.233)
Examples:
The Olivine group represents a complete solid solution series
Compositions range from a 100% Mg-rich "end member" (forsterite) to a 100%
Fe-rich "end member" (fayalite), with all mixtures of these two elements possible (e.g.,
90% Mg and 10% Fe)
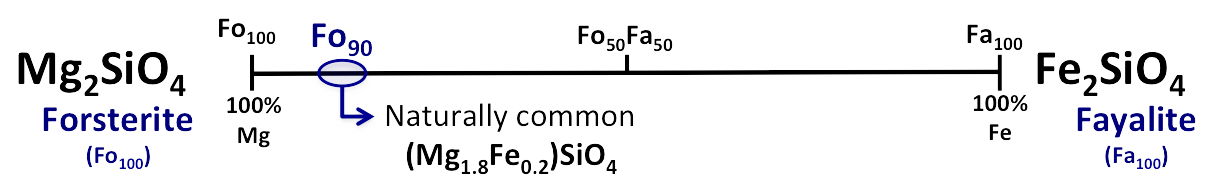
- There is a complete solid solution series between the endmembers of this series because Fe and Mg have same charge and
similar ionic radii
Plagioclase Feldspars also display a complete solid solution series ranging from
a 100% Na-rich end member (albite) to a 100% Ca-rich end member (anorthite),
with all intermediate compositions possible.
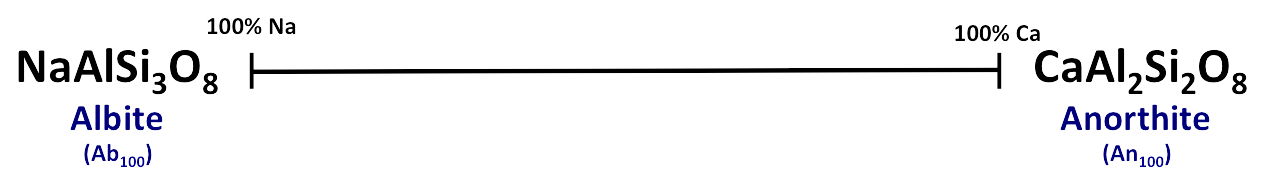
- Na and Ca are close enough in size to freely substitute but because Na has a charge of +1 and Ca has a charge of +2, a double substitution must occur to maintain the
charge balance. The other cations in the feldspar composition are Al
(charge = +3) and Si (charge = +4). (Al and Si are also close enough in size for
free substitution to occur) For each substitution of Ca (+2) for Na (+1),
an equal amount of Al (+3) is substituted for Si (+4)
- In contrast Alkali Feldspars display only a limited solid solution
series because the radius of K (1.33 Å) is ~37% larger than
that of Na (0.97 Å)
Some carbonates show a limited solid solution series (e.g., calcite & magnesite)
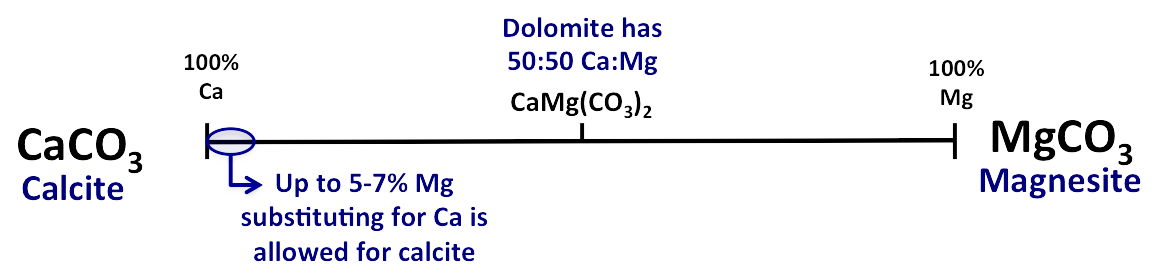
- to maintain the calcite-type structure, large cations (e.g., Ca
radius = 1.08 Å) cannot be freely replaced by small ones (e.g., Mg
radius = 0.66 Å); if too much Mg is substituted, the structure destabilizes and collapses
- dolomite: 50/50 mix of Ca and Mg
*structurally completely different
from calcite
- has alternating Ca-rich and Mg-rich layers
|
http://classes.colgate.edu/rapril/geol201/
Questions to:
rapril@mail.colgate.edu
Copyright 1997 © Colgate University.
|
|




