ev = valence # of cation / coordination # of cation
EXAMPLE 1:
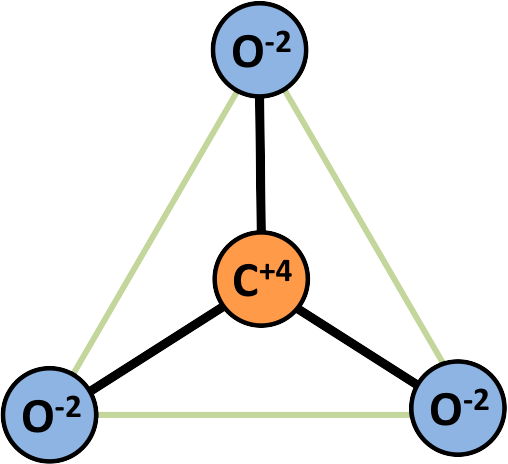
ANISODESMIC Bonding in Carbonates - Calcite:
Anisodesmic: bond strength is unevenly distributed among cations
⋅ ev > ½ the valence of the anion
Ca[CO32-] (example of CO3 radical in carbonate mineral)
CO32- (triangular coordination polyhedron)
ev = 4/3 which is greater than ½ the valence of the anion
|
|
- C has a +4 charge; O has a -2 charge; Ca has a +2 charge
- in the CO3 radical, 1⅓ of a negative charge is contributed by each of the 3 oxygens (net -4 charge) to balance the +4 charge of the carbon
- the residual -⅔ charge on each of the 3 oxygens (net charge -2) balances with the +2 charge of the Ca
- the greater charge allocated to the carbon makes the bond of oxygen to carbon stronger than bond of oxygen to Ca2+
- this is an important factor in weathering
EXAMPLE 2:
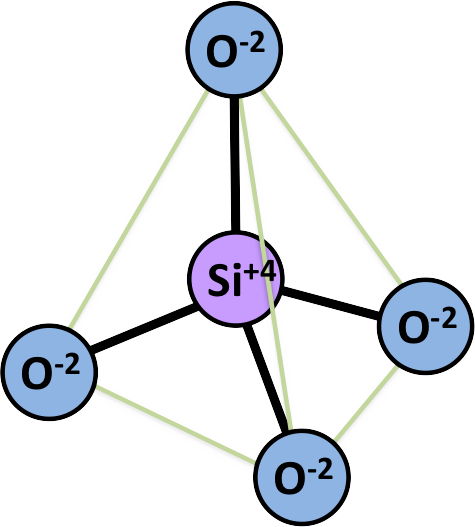
MESODESMIC Bonding in Silicates - Olivine:
Mesodesmic: ev = ½ the valence of the anion
Mg2[SiO44-] (example of SiO4 radical in silicate mineral)
SiO42- (tetrahedral coordination polyhedron)
ev = 4/4 which = ½ the valence of the anion
|
|
- Si has a +4 charge; O has a -2 charge; Mg has a +2 charge
- in the SiO4 radical, 1 negative charge is contributed by each of the 4 oxygens (net -4 charge) to balance the +4 charge of the Si
- the remaining -1 charge on each of the 4 oxygens (net charge -4) balances with other cations in the structure (ex. the +4 charge of the Mg ions)
- the same charge is allocated by the oxygens to the Si and the Mg
- this type of charge balance leads to the linkages between Si tetrahedron (for example chain, ring, and sheet structures) on which the
silicate subclasses are based
EXAMPLE 3:
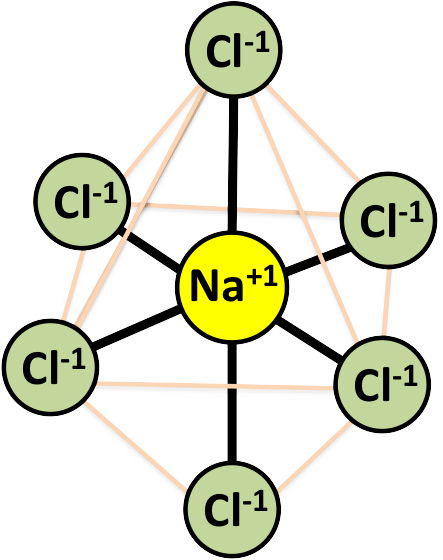
ISODESMIC Bonding in Halides - NaCl (Halite):
Isodesmic: ev < ½ the valence of the anion
|
each Na cation is surrounded by 6 Cl in an octahedral coordination polyhedron
ev = 1/6 which < ½ the valence of the anion
|
|
|
http://classes.colgate.edu/rapril/geol201/
Questions to:
rapril@mail.colgate.edu
Copyright 1997 © Colgate University.
|
|




