
Week of April 19 - April 22, 2010

Qualitative Analysis (Exp. 21-116) Report Sheets are due this week.
EXPERIMENT 22-026: Investigation of Electrochemical Cells
![]() The purpose of the final laboratory experiment of the semester is to construct and examine 10 electrochemical (galvanic) cells. You will investigate several oxidation-reduction (or redox) reactions. These redox reactions involve the transfer of electrons from the species being oxidized to the species being reduced. Remember that redox reactions must occur in pairs (i.e. oxidation and reduction always occur together)
The purpose of the final laboratory experiment of the semester is to construct and examine 10 electrochemical (galvanic) cells. You will investigate several oxidation-reduction (or redox) reactions. These redox reactions involve the transfer of electrons from the species being oxidized to the species being reduced. Remember that redox reactions must occur in pairs (i.e. oxidation and reduction always occur together)
An example of an electrochemical cell is shown below:
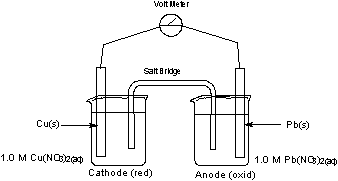
Figure 1. A electrochemical cell constructed from Pb and Cu(NO3)2 (25 °C, 1 atm).
Another way to represent Figure 1 is to use the following convention to describe the electrochemical cell: Pb(s)/Pb2+(aq)//Cu2+(aq)/Cu(s)
If the oxidation and reduction half-reactions were allowed to occur in the same container, the energy associated with the oxidation-reduction reaction would simply be released as heat. However, if the half-reactions were separated, as in Figure 1, and the electrons were forced to flow through a wire from the substance being oxidized (Pb) to the substance being reduced (Cu2+), the flowing electrons could be utilized to carry out useful work. The capacity of these flowing electrons to do work is manifested in their electromotive force (emf), or potential. A measure of the emf is easily obtained by measuring the voltage of the two half-reactions. In this experiment you will construct several such cells, using different substances, and measure the voltage between the cells.
By convention, emf values for half-reactions are expressed for the reduction reaction. This emf value is referred to as the reduction potential. Reduction potentials for various half-reactions can be compared under the same conditions of concentration (1 M for dissolved reactants and products), gas pressure (1 atm for all gaseous reactants and products), and temperature (25 °C). Such reduction potentials are termed standard reduction potentials, and are usually denoted by e°.
![]() The Nernst Equation
The Nernst Equation
The concentration of reactants and products affect the observed emf of a galvanic cell; the Nernst equation describes the relationship between the concentrations and the resulting voltage:
![]()
where R is the Ideal Gas Law Constant (8.314 Jmol-1K-1), T is the temperature (in Kelvin), n is the moles of electrons involved in the reaction, F is the conversion factor between coulombs and moles of electrons (96,485 Cmol-1). Q, the reaction quotient for the reaction involved, is the mass action expression from the reaction. Plugging the values for R and F into the Nernst equation, and keeping things at 25 °C, gives:
![]()
Consider the example given in Figure 1.
Type of Reaction |
Reaction |
e°(V) |
| Oxidation (anode) | Pb -----> Pb2+ + 2e - | 0.130 |
| Reduction (cathode) | Cu2+ + 2e - -----> Cu | 0.340 |
| Net | Pb + Cu2+ -----> Pb2+ + Cu | 0.470 |
The standard reduction potential (e°) for this reaction is calculated to be 0.47 V. In order to find the reduction potential of the cell (e), we have to use the Nernst equation. For the example described in figure 1, Q = 1.
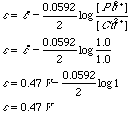
![]() Concentration Cells
Concentration Cells
The Nernst equation predicts that it is possible to construct an electrochemical cell in which the same half reaction occurs in both compartments. Suppose a cell is constructed like that of figure 2 which contains 1 mol/L Au3+ in one compartment and 0.1 mol/L Au3+ in the other compartment.
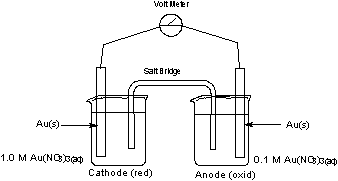
Figure 2. A concentration cell composed of 0.10 M and 1.0 M Au3+
Type of Reaction |
Reaction |
e°(V) |
| Oxidation (anode) | Au -----> Au3+ (0.10 M) + 3e - | -1.50 |
| Reduction (cathode) | Au3+ (1.0 M) + 3e - -----> Au | 1.50 |
| Net | Au3+ (1.0 M) -----> Au3+ (0.10M) | 0 |
Using the Nernst Equation, we can determine e for this cell.
![]() Experimental.
Experimental.
You will construct the 9 electrochemical cells described in Table 1 in the lab manual (p. 131) and measure the voltage of each of these cells. For the first four cells you are asked to compare the potential the Nernst equation predicts to the experimentally determined potential. For cells 5 - 7 you are asked to calculate the expected voltage for each concentration cell using the Nernst equation and to compare the calculated value with the experimentally determined values. For cell 8, you will determine a Ksp. For cell 9, you will determine a Keq. Finally, for cell 10 you are asked to construct an electrochemical cell to determine the standard potential (e°) expected for the given chemical reaction. You will find a table of standard reduction potentials in Appendix C of your lab manual (p. 146).
This lab will be due at the end of the lab period.
Report Sheets for Experiments 22-026: Acrobat Reader is required for this file.
![]() Chemistry 102 Laboratory Home Page
Chemistry 102 Laboratory Home Page
Last Updated: