

This lab is due the week of October 26, 2009.
Experiment 6-085 Spectroscopic Analysis of the Active Ingredient in Aspirin
Report Sheet for experiment 6-085: Extra report sheet (pdf format).
The use of willow bark to cure "the ague" was described in the literature as early as 1763. About 1860, the active ingredient , salicylic acid, was isolated from a different source, Spiraea ulmaria, the meadowsweet flower. In 1893 a German chemist from the Bayer chemical firm made the drug a commercial success by adding the acetyl group (-COCH3) to the salicylic acid to improve its effectiveness and to reduce its harsh taste and erosive behavior. The trade name "aspirin" under which Bayer marketed this familiar and remarkably effective drug, is derived from the words acetyl and Spiraea mulmaria.
The Chemistry:
The reaction for the acetylation of salicylic acid is shown in Equation 1. When acetylsalicylic acid undergoes hydrolysis, the acetylation process is reversed, as in Equation 2. This reaction often occurs when aspirin tablets are stored in a moist environment; the characteristic vinegar odor of acetic acid can be detected when aspirin is old and moist. The hydrolysis is accelerated in the presence of acids or bases.
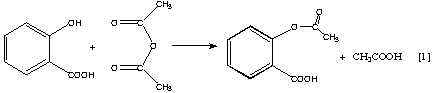
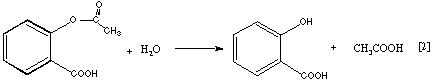
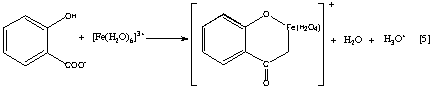
The most common method of determination of acetylsalicylic acid involves its quantitative hydrolysis in a basic medium to yield the salicylate dianion (Equation 3), acidification to the monoanion (Equation 4), and finally complexation with Fe(III) to yield the brightly colored tetraaquosalicylatoiron(III) complex (Equation 5).
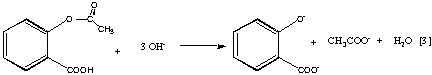
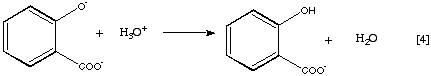
The Experiment:
![]() In
this experiment, an aspirin sample is weighed, hydrolyzed with
dilute NaOH, transferred quantitatively to a volumetric flask,
and diluted to a known volume. An aliquot of this solution is
then diluted to a known volume with a buffered solution of
iron(III) chloride at a pH of 1.6. The same chemical procedure,
starting with a standard, pure acetylsalicylic acid is used to
prepare five solutions of known concentration which constitute a
standard series. A reagent blank is similarly prepared to correct
for impurities or interferences in the chemicals used. The amount
of acetylsalicylic acid in each sample will then be estimated by
spectrophotometric analysis.
In
this experiment, an aspirin sample is weighed, hydrolyzed with
dilute NaOH, transferred quantitatively to a volumetric flask,
and diluted to a known volume. An aliquot of this solution is
then diluted to a known volume with a buffered solution of
iron(III) chloride at a pH of 1.6. The same chemical procedure,
starting with a standard, pure acetylsalicylic acid is used to
prepare five solutions of known concentration which constitute a
standard series. A reagent blank is similarly prepared to correct
for impurities or interferences in the chemicals used. The amount
of acetylsalicylic acid in each sample will then be estimated by
spectrophotometric analysis.
![]() Students will need to prepare a standard series of solutions of
acetylsalicylic acid (ASA) and individual samples of aspirin.
Students should work with a partner to prepare the standard
series and then work independently to prepare separate soft drink
samples.
Students will need to prepare a standard series of solutions of
acetylsalicylic acid (ASA) and individual samples of aspirin.
Students should work with a partner to prepare the standard
series and then work independently to prepare separate soft drink
samples.
Students will use volumetric glassware to prepare their solutions for analysis. Analysis will involve the use of spectroscopy. There is a section on Spectrophotometers and Spectroscopy in the laboratory manual (p. 44). Students should read this section carefully before coming to lab.
Once a standard absorbance curve has been generated, one can find the concentration of acetylsalicylic acid (or in reality salicylic acid) in an aspirin sample by employing Beer's law:
![]()
![]()
Beer's Law gives us an equation of a straight line (y = mx + b) with the y-intercept (b) = 0. In this experiment our standard curve is a straight line (or should be), so we can equate slope of the standard curve with ![]() . We will use 1.00 cm path length cuvettes for the spectrophotometric analysis of our acetylsalicylic acid standard soltuions and aspirin samples (thus l = 1.00 cm) and so
. We will use 1.00 cm path length cuvettes for the spectrophotometric analysis of our acetylsalicylic acid standard soltuions and aspirin samples (thus l = 1.00 cm) and so ![]() will simply equal the slope of the standard curve. We will measure the absorbance (A) of the aspirin samples and then solve for the concentration (c) of salicylic
acid in the aspirin samples. From this data, you can then
calulate the mg of active ingredient in 1 aspirin tablet.
will simply equal the slope of the standard curve. We will measure the absorbance (A) of the aspirin samples and then solve for the concentration (c) of salicylic
acid in the aspirin samples. From this data, you can then
calulate the mg of active ingredient in 1 aspirin tablet.
Dr. J's white board notes: Includes sample calculations (pdf format).
Graphing with E xcel: (pdf format)