
Colgate University First Year Seminar 39: Earth Resources
Term papers
________________________________________________________
The Problem of Smog in the Lower Fraser Valley
Mary Benton Curtis
December, 2000
Introduction
Smog is a popular name for the brownish yellow haze that hangs in the warm, still air over many cities (see figure 1). The two key components of smog are fine, airborne particles and ground level ozone. Airborne particles are made up of dust or droplets of liquid that are small enough to remain suspended in the air. The primary fine particles are released directly into the air from sources such as automobile tailpipes. Secondary particles are formed in the air from physical and chemical reactions that involve the emission of gases. (What is Air Pollution? 1995). Fine and secondary particles are what give smog its color. Depending on the types of particles present, smog can appear yellowish-brown, or even white.
Smog’s Relation to Ozone
Smog is also made up, in large part, by ozone gas (O3), which forms in the atmosphere when three atoms of oxygen are combined. Ozone has the same chemical structure whether it occurs high above the earth or at the ground level and can be "good" or "bad," depending on its location. Ozone occurs in two layers of the atmosphere (see figure 2). The stratosphere or "good" ozone layer extends upward from about 10 to 30 miles and protects life on earth from the sun’s harmful ultraviolet rays, UV-b. The layer surrounding the earth’s surface is known as the troposphere and this is where harmful ground level ozone is produced (Good up high, bad nearby, 1998). Ground level ozone does occur naturally from human's usage of fossil fuels, but only in very small amounts. Smog is usually created when certain pollutants are mixed. The most important pollutants that contribute to smog are nitrogen oxides and volatile organic compounds. Nitrogen oxides, also known as N0x, are produced when fossil fuels, like gasoline, natural gas, heating oil and coal are burned (Smog an Indicator, 1999). They also occur naturally in the environment as a result of forest fires, volcanoes and the soil. The volatile organic compounds, or VOC’s, mainly come from the evaporation of liquid fuels, solvents and organic chemicals such as nail polish remover, paints and cleaners (Smog an Indicator, 1999). Trees in cities and forests produce VOC’s naturally but only in very small amounts.
Factors that Affect Smog
Weather, climate and surfaces also affect smog. Smog levels are generally more prevalent on hot, sunny summer days because smog-forming reactions depend on temperature and sunlight (Reducing Smog, 1998). These periods of intense smog can last up to several days in one area. Both rain and wind can help reduce smog levels. Rain can clear the air of the pollutants that cause the smog but at the same time this also creates acid rain including sleet, hail and snow that is acidic (Britannica, 2000). Acidic precipitation, or acid rain, has a pH lower than 5.0. The formation of acid rain depends on is N0x, which is one of the same agents that causes smog (Reducing Smog, 1998). Wind also helps reduce smog by blowing it away and dispersing it. The ground level ozone collects over cities that are producing the N0x and the VOC, but then can migrate several hundred kilometers downwind. If the pollutants travel to cities that are surrounded by hills or mountains, the airflow gets trapped and that increases the smog level even more (Smog an Indicator, 1998). For example, this often occurs in the Lower Fraser Valley, which is located in Canada (see figure 4).
The Formation of Smog in the Lower Fraser Valley
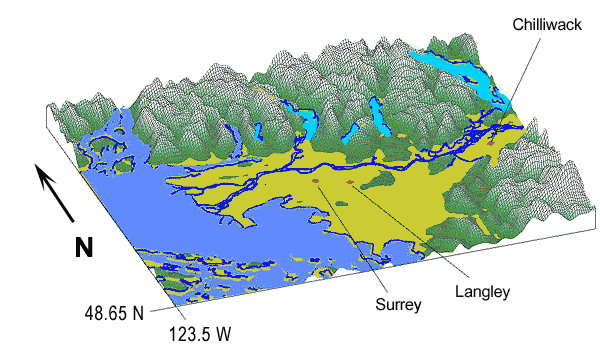
The Lower Fraser Valley, which is in the British Columbia providence, is an area surrounded by the Coastal Mountains to the north and the Cascade Mountains to the southeast. These geographical features in addition to the summer breezes off the straight of Georgia, restrict airflow patterns and contributes to the smog problem (Smog an Indicator, 1998). Summer weather conditions and pollutants from human activities are the main factors that permit the formation and accumulation of air pollution (Smog an Indicator, 1998). The worst air quality usually occurs during the summer when there are clear skies and warm temperatures. A warm layer of air may rest over the cooler and denser air that lies beneath it and it forms a condition known as atmospheric inversion (Craig, 1998). These conditions are favorable at the end of a sunny day when the air near the ground starts to lose heat rapidly. Sometimes these conversions can last several days. The N0x and V0C cannot penetrate above the inversion and they become trapped and affect the area with a dense smog. The temperature inversions that occur in the Lower Fraser Valley trap the pollutants near the ground and then light winds transport them to the valley where they undergo a photochemical reaction to form smog. (Smog, 1998).
Sources of Smog in the Lower Fraser Valley
Most of the smog in the Lower Fraser Valley area is generated locally. The major sources of VOC’s come from cars, the evaporation of gasoline from gas pumps and from solvents (Smog an Indicator, 1998). 80% of all the pollutants contributing to the ground level ozone in the Lower Fraser Valley are from cars (Smog an Indicator, 1998). The Lower Fraser Valley is experiencing a rapid population growth, which contributes to an increase in the number of cars (see graph 1). There were 1.2 million registered cars on the road in 1999 and that number is expected to increase by 60% over the next twenty years (Smog an Indicator, 1998). The N0x produced in the area are mainly from industries and factories that burn fuels (Smog an Indicator, 1998).
Smog’s Impact on Health
Smog is detrimental to humans, plants and animals as well as the economy. Smog is extremely dangerous to human health. Low concentrations of ground level ozone can irritate the eyes, nose and throat. As smog increases it can also trigger more serious health problems including: asthma, bronchitis, coughing and chest pains, increased susceptibility to respitory infections, decreased lung function and physical performance (Smog, Who Does it Hurt? 1999). Prolonged exposure can eventually damage lung tissue, cause premature aging of the lungs and contribute to lung disease ( Smog, Who does it Hurt?, 1999), (see figure 2). Children, the elderly and those with impaired lung function are considered to be at the most risk. In 1995, a UBC department of Medicine researcher found that smog caused 82 premature deaths, 146 hospitalizations due to asthma and 354 extra emergency room visits in the Lower Fraser Valley (Smog, Who does it Hurt?, 1999).
Smog’s Impacts on Plants and Animals
Smog also causes problems for plants and animal life. Decreased lung capacity and lung elasticity affect the animals as a result of smog. It also decreases the lung's ability to ward off diseases (Smog, 1996). As for plants, smog damages the leaf tissues that affect the plants ability to grow. Visible damage to the leaf tissue includes discoloration, black and white spots, paper-thin areas on the leaf and leaf loss as well. 10 to 40% of the growth can be lost to a plant because of smog (Smog, 1996). Excessive smog levels have also been linked to the decline of several Canadian forest areas. Current agricultural losses are estimated to be $ 9 million in the Lower Fraser Valley (Smog, 1996).
Smog’s Impact on the Economy
Smog also has a major impact on the economy. First of all, the health problems caused by smog lead to higher health care costs. Lower Fraser Valley annual health care costs due to smog were estimated to be between $420 to $900 million (Smog an Indicator, 1998). Secondly, as mentioned above, the crop damage is quite costly. Also smog damages synthetic materials, causes cracks in rubber and accelerates the fading of dyes and the deterioration of paints (Smog, 1996). Damage done to materials exposed to the pollutants have been estimated up to $1 billion in the Lower Fraser Valley (Smog, 1996). In 1994, a study of the Lower Fraser Valley found that the particle pollution was responsible for visibility frequently being degraded below public acceptance (Let's Clear the Air, 1996). Visibility conditions, caused by the deterioration of paint, affect views in the Valley which impact tourism. This influences economics in the Lower Fraser Valley area. Another economic problem is the detrimental effect smog has on the Red Sugar Maple tree. Canada’s forestry industry depends on this tree to be successful.
Smog Levels
In Canada, smog levels are measured against air quality objectives developed by the federal and provincial governments. Their aim is to protect the health of humans, crops and forests by specifying target levels for key air pollutants (The Green Lane, 1996). The current air quality objective for ground level ozone in Canada is 82 ppb ( parts per billion) over a one-hour time period. Between 1984 and 1991, this level was exceeded at least once in almost every major Canadian city (The Green Lane, 1996). Around the Lower Fraser Valley, especially in the summer, concentrations of ozone have been more than twice the air quality objective level of 82 ppb. Health studies have also focused on two groups of airborne particles that are also dangerous to human health. These fine particles were so small that they were able to remain suspended in the air where they can be inhaled and deposited into the lungs (The Green Lane, 1996). The first type are the PM10, which are particles less than 10 microns across. These particles measure about 1/8 of the width of a human hair and are composed of soil, soot and dust from construction and roadways (Environmental Protection, 1995). The second type are the PM2.5, which are particles less than 2.5 microns across (Environmental Protection, 1995). Most of the secondary particles are PM2.5 and these cause the most concern; they penetrate deep into the lungs, where they cause irritation and can cause lung disease. In the Lower Fraser Valley, diesel and gasoline vehicles are the major sources of PM2.5 (The Green Lane, 1996). These are mainly formed from N0x and VOC (see graph 3).
Reducing Smog Levels
A wide range of initiates are underway to help reduce smog. The Canadian Clean Air Act Amendments of 1990 require cities to enforce programs to further reduce emissions of ozone precursors from sources such as cars, fuels, industrial facilities, power plants and consumer/commercial products (Smog Let's Clear the Air, 1998). Power plants will be reducing emissions, cleaner cars and fuels are being developed, many gas stations are using special nozzles at the pumps to recapture gasoline vapors and vehicle inspection programs are being improved under the Air Quality Management Plan to reduce emissions (Smog an Indicator, 1999). Minor lifestyle changes can also help reduce the amount of smog. Using public transit or becoming a part of a carpool is extremely helpful because cars are the main source of the pollution causing smog. Also, when people are looking for new cars they should consider buying one that is smaller and fuel-efficient. Preventing gas leaks by not over filling the gas tank is also important because spilled gas emits VOC’s (Reducing Smog, 1998). Using garden tools that do not run on gasoline is also helpful and trying to avoid lighter fluid is important as well because it releases VOC’s. Painting with water based paints is helpful because the oil based ones contain three to five times more toxic solvents than the water based paints (Let's Clear the Air, 1998). Lastly, sealing containers of household cleaners, workshop chemicals and solvents helps to prevent VOC’s from evaporating into the air.
New Innovations for Reducing Smog
New experiments are being conducted to reduce the levels of smog. Calculations show that on certain days, given the right wind speed and temperature, bellows can create air currents that will sweep the smog out to sea. Tests with one bellow created a bubble of fresh air over ten blocks. It has been estimated that the use of 300 bellows placed around a city would clean the air. The bellows are environmentally friendly. They are opened and closed by a ten-person team pulling on chains connected to an assortment of gears and pulleys. There is one draw back. A 50-mile per hour wind is generated for 20 minutes. A poll showed that 90% of people would be willing to put up with the occasional gusts (Wilson, 1998). The other idea under way involves the use of helium balloons. The helium balloon would be coupled with a large cylinder that could restore normal convection, clearing the smog out of the area (McWilliams, 1998).
Figure 1

Smog is the popular name for the brownish yellow haze that hangs over many cities.
(http://www.on.ec.gc.ca/glimr/data/climate-flyer/smog.html)
Graph 1
Figure 2
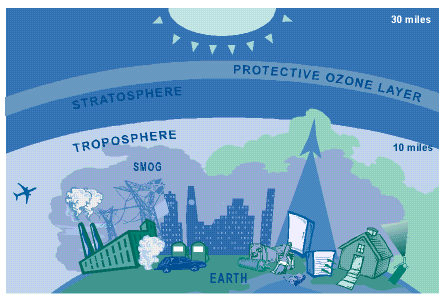
This figure shows the position of the stratosphere in relation to the troposphere.
(http://www.epa.gov/oar/oaqps/gooduphigh/#what)
Figure 3
A B
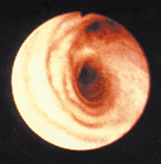
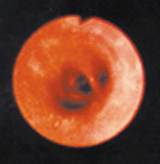
This photo shows a healthy lung air way (A) and an inflamed lung air way (B). Ozone can inflame the lung's lining, and repeated episodes of inflammation may cause permanent changes in the lung.
(
http://www.epa.gov/airnow/health/smog1.html#1)
Graph 2
(
http://www.env.gov.bc.ca/sppl/cvf/cleanv.html)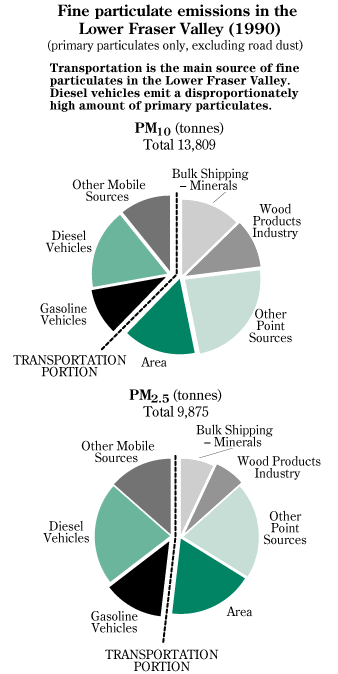
Figure 4
The Lower Fraser Valley is located in British Columbia, Canada
(http://www.ecoinfo.org/env_ind/region/smog/smog_data.htm#Map)
Bibliography
Craig, J., Skinner, B., Vaughan, D. ( 1988). Resources of the Earth. New Jersey:
Prentice Hall.
McWilliam, F. (1998). Eye On The World. Geographical,70, 38-39.
No Room to Breathe: Photochemical Smog and Ground Level Ozone [online]
Available: http://www.elp.gov.bc.ca/epd/epdpalar/vehicle/nrtbsag.html
[1992, August].
Ozone: Good up High, bad nearby [online] Available:
http://www.epa.gov/oar/oaqps/gooduphigh. [1997, October].
Reducing Smog [online] Available:
http://www.ns.ec.gc.ca/epb/ccme/smog.html [ 1998]
Smog Lets Clear the air [online] Available:
http://www.on.ec.gc.ca/glimr/data/climate-flyer/smog.html [ February, 1996]
Smog over the Lower Fraser Valley [ online] Available:
http://www.ecoinfo.org/env_ind/region/smog/smog.htm. [ 2000, September].
The Green Lane [online] Available:
http://www.ec.gc.ca/press/smog2_b_e.htm. [November 1997].
What is air pollution [Online] Available:
http://www.env.gov.bc.ca/sppl/cvf/effects.html [ 1995]
Wilson, H. (1998). You don’t need a weatherman… Discover,19, 20.