
March 29 - April 1, 2010

Experiment 20-050: Determination of pKa
![]() In this experiment, a sample of one of the weak acids listed in Table 1 will be analyzed. You will perform a potentiometric titration from which a molecular weight and a pKa value will be obtained. These two pieces of information should allow the correct identification of the unknown weak acid sample.
In this experiment, a sample of one of the weak acids listed in Table 1 will be analyzed. You will perform a potentiometric titration from which a molecular weight and a pKa value will be obtained. These two pieces of information should allow the correct identification of the unknown weak acid sample.
Table 1: Weak Acids Used as Unknowns in Experiment 20-050
|
Weak Acid |
Formula |
Molar mass (g/mole) |
pKa |
|
Ascorbic |
C6H8O6 |
176.12 |
4.17 |
|
Nicotinic |
C6H5NO2 |
123.11 |
4.85 |
|
Potassium Hydrogen Phthalate |
HC8H4O4K |
204.23 |
5.41 |
|
Benzoic |
C7H6O2 |
122.12 |
4.19 |
Molecular Weight
![]() All of the acids in Table 1 are monoprotic ( i.e. it takes only one mole of NaOH to neutralize one mole of the weak acid). Thus, the titration of a carefully weighed sample of weak acid with the standardized NaOH solution allows the calculation of the molecular weight of the weak acid. At the neutralization point, the moles of NaOH will equal the moles of acid. The moles of NaOH in the reaction can be determined if the molarity of the OH(aq) and the volume of OH(aq) added are known. If the moles of base are known, then the moles of acid is also known, and knowing the grams and moles of acid involved in the reaction, the determination the molecular weight is straightforward.
All of the acids in Table 1 are monoprotic ( i.e. it takes only one mole of NaOH to neutralize one mole of the weak acid). Thus, the titration of a carefully weighed sample of weak acid with the standardized NaOH solution allows the calculation of the molecular weight of the weak acid. At the neutralization point, the moles of NaOH will equal the moles of acid. The moles of NaOH in the reaction can be determined if the molarity of the OH(aq) and the volume of OH(aq) added are known. If the moles of base are known, then the moles of acid is also known, and knowing the grams and moles of acid involved in the reaction, the determination the molecular weight is straightforward.
Estimation of pKa
![]() The pKa of the weak acid can be estimated from the titration curve by reading the pH when the acid is exactly one-half titrated. At that point the concentration of HA and its conjugate base, A-, are equal, and the Henderson-Hasselbalch equation::
The pKa of the weak acid can be estimated from the titration curve by reading the pH when the acid is exactly one-half titrated. At that point the concentration of HA and its conjugate base, A-, are equal, and the Henderson-Hasselbalch equation::
![]()
indicates that the pH = pKa at that point. Analysis of the pH-titration graph will allows the determination of the pH when the volume of NaOH(aq) added is exactly half the amount needed to reach the equivalence point. Alternatively, a solution of weak acid can be prepared and half neutralized by adding the appropriate amount of base. The pH can then be measured directly. Both methods will be done in this laboratory.
A typical pH-titration curve for the weak acid, HF (0.200 M), titrated wih NaOH (0.150 M) is shown below:
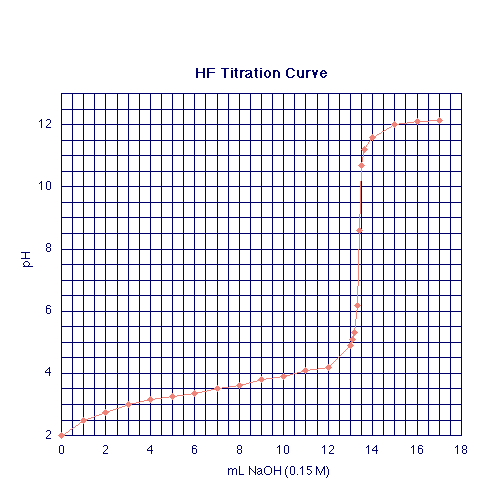
Titration of 10.00 mL of 0.200 M HF with 0.150 M NaOH
According to the Henderson-Hasselbalch equation, when [F-(aq)] = [HF(aq)], the pH of the solution will equal the pKa of the titrated weak acid. In this case, this occurs when exactly 1/2 of the HF is neutralized, or at 1/2 of the NaOH volume required to reach the neutralization point. By definition the equivalence (neutralization) point of this titration occurs when moles OH-(aq) added equal moles of HF initially present. As you can read from the graph, this occurs at 13.33 mL of NaOH added. Hence, [F-(aq)] = [HF(aq)] at 6.665 mL NaOH added, and pKa of HF is 3.45. You can then calculate the Ka for HF to be ~3.54 x 10-4 (i e. 10-3.45).
Your tasks for experiment 20-050:
![]() You will determine the MW of your weak acid sample by making up a standard solution (known amount in a known volume) of the acid and subsequently titrating a known volume of your acid with a standardized NaOH solution. You should repeat this titration until you have three trials within 0.05 mL.
You will determine the MW of your weak acid sample by making up a standard solution (known amount in a known volume) of the acid and subsequently titrating a known volume of your acid with a standardized NaOH solution. You should repeat this titration until you have three trials within 0.05 mL.
![]() You will then perform a potentiometric titration (you will measure the pH of your solution with a pH meter as you titrate) of your acid sample and generate a pH-titration curve similar to the HF example shown above. From the pH-titration curve you will determine the pKa and subsequently the Ka of your weak acid. You will also be asked to identify the acid from the information given in Table 1.
You will then perform a potentiometric titration (you will measure the pH of your solution with a pH meter as you titrate) of your acid sample and generate a pH-titration curve similar to the HF example shown above. From the pH-titration curve you will determine the pKa and subsequently the Ka of your weak acid. You will also be asked to identify the acid from the information given in Table 1.
![]() Finally, you will directly measure the pKa of your weak acidby taking a known volume of your acid and adding 1/2 the NaOH volume required to reach the neutralization point (you will determine this volume from your pH-titration curve) and then measuring the pH of the solution with a pH meter. This should be repeated at least three times.
Finally, you will directly measure the pKa of your weak acidby taking a known volume of your acid and adding 1/2 the NaOH volume required to reach the neutralization point (you will determine this volume from your pH-titration curve) and then measuring the pH of the solution with a pH meter. This should be repeated at least three times.
Report Sheets for experiment 20-050: Acrobat Reader is required for this files
![]() Chemistry 102 Laboratory Home Page
Chemistry 102 Laboratory Home Page
File Updated: