
Potentiometric Determination of pKa and Molecular Weight of a Weak Acid.
In this example, a 0.3400 g sample of a monoprotic unknown acid was quantitatively transferred to a 50.0 mL volumetric flask, approximately 10 mL of 95 % aqueous ethanol and 10 mL of distilled water were added to the flask. The solution was mixed to dissolve the solid, and then brought to volume with distilled water and mixed well. The data for the titration curve shown below (Figure 1) was generated by titrating a 5.00 mL sample of the unknown acid with a 0.04924 M NaOH solution. The NaOH was added using a 10.00 mL volumetric buret and the pH was recorded on a standard pH meter that had been calibrated between pH 4 and pH 7 at 23 oC.
Figure 1: pH Experimentally Determined Titration Curve for a Monoprotic Weak Acid
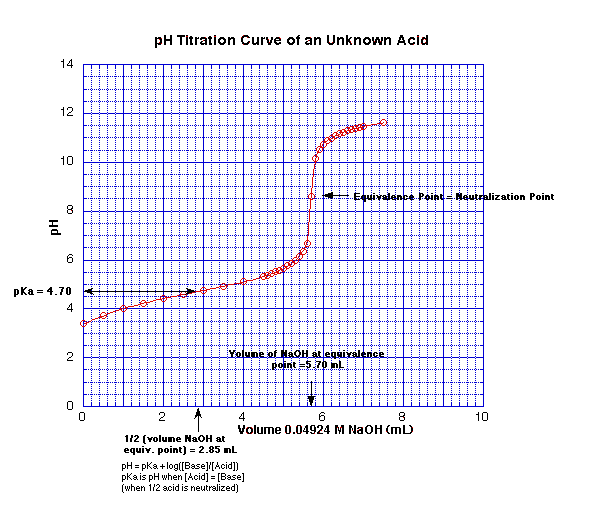
Estimation of pKa:
As shown in Figure 1, the volume of NaOH at the estimated equivalence point is 5.70 mL. The pKa was estimated to be 4.70. This estimation was done by reading the pH at a volume of 2.85 mL (1/2 the volume required to reach the equivalence point).
Direct Measurement of pKa:
Direct measurement of the pKa was done by adding 2.85 mL of NaOH to a 5.00 mL sample of the acid, mixing and measuring the pH with a standard pH meter. This was repeated twice. The directly measured values are 4.80, 4.75 and 4.83, respectively. The average value of the directly measured pKa is 4.79.
Calculation of Molecular Weight of the Unknown Acid:
Since the unknown acid is monoprotic, we can assume that the moles of base = moles of acid at the equivalence point:
0.0494 mole NaOH/L * 0.00570 L = 2.81 x 10-4 mole NaOH = 2.81 x 10-4 mole acid
We know the mass of the unknown acid in 50.0 mL (0.3400 g, in this example) and we know that we titrated a 5.00 mL sample of the acid:
0.3400 g acid /50.0 mL = x /5.00 mL
x = (0.3400 g Acid)(5.00 mL/50.0 mL)
x = 0.0340 g acid
We have grams of acid and moles of acid, so we can calculate molecular weight of the unkown acid:
0.0340 g acid/2.81 x 10-4 mole acid = 121 g/mole acid
Now that we have an estimated pKa, 4.70, a directly measured pKa, 4.79 (average of 3 trials) and a calculated molecular weight, 121 g/mole. We can identify our unknown acid from the data given in Table 1 (see below or on p. 101 of the lab manual) as Nicotinic Acid.
Table 1: Molecular Mass and pKa of Unknown Acids
Weak Acid |
Formula |
Molar mass (g/mole) |
pKa |
Ascorbic |
C6H8O6 |
176.12 |
4.17 |
Nicotinic |
C6H5NO2 |
123.11 |
4.85 |
Potassium Hydrogen Phthalate |
HC8H4O4K |
204.23 |
5.41 |
Benzoic |
C7H6O2 |
122.12 |
4.19 |
![]() Chemistry 102 Laboratory Home Page
Chemistry 102 Laboratory Home Page
Last Updated: