

Experiment 9-016: Molecular Models
Report Sheet for experiment 9-016: Extra report sheet (pdf format).
![]() A fundamental tenet of chemistry is that structure is related to function. That is, the chemical behavior of a molecule is determined by its structure. Recognizing that, it is apparent that understanding (and being able to predict) the structure of a molecule is crucial to understanding its chemistry. While molecules are often represented on 2-dimensional surfaces (textbook pages, blackboards, etc.), molecules actually exist in a 3-dimensional world. This “lab” is designed to help you visualize the 3-dimensional structures of molecules.
A fundamental tenet of chemistry is that structure is related to function. That is, the chemical behavior of a molecule is determined by its structure. Recognizing that, it is apparent that understanding (and being able to predict) the structure of a molecule is crucial to understanding its chemistry. While molecules are often represented on 2-dimensional surfaces (textbook pages, blackboards, etc.), molecules actually exist in a 3-dimensional world. This “lab” is designed to help you visualize the 3-dimensional structures of molecules.
For this laboratory exercise, you are to build some molecular models, study the factors which determine their shapes, and then predict the structure and polarity of the actual molecules. Bring your molecular model kit with you to the laboratory.
In brief the shape of a molecule is determined by the distribution of the electrons in the molecule. That means you must become aware of electrons, especially the valence electrons. This requires a crucial shift in thinking, for when we write a molecular structure, we generally just show the positions of the nuclei; we seem to ignore the electrons. When we say that CO2 is linear, we mean that the three nuclei the C between the two O's all line up (OCO); when we say that the H2O molecule is bent, we are referring to the fact the three nuclei do not lie in a straight line (H O H). There is no mention of electrons in these structural descriptions just nuclei. Ironically, the key to understanding where the nuclei are is to focus on the electrons, not the nuclei.
![]() The Chemistry (theoretically):
The Chemistry (theoretically):
Lewis Diagrams
To help predict bonding patterns in simple molecules, chemists generally use the simple, yet powerful model proposed by Professor G.N. Lewis (California Institute of Technology) in 1923. Lewis proposed the "octet rule", in which atoms form bonds by losing, gaining or sharing enough valence electrons to obtain the same number of electrons as the nearest noble gas. The octet rule is remarkably valid for molecules formed from elements in the first, second and third rows of the periodic table (H through Cl).
The VSEPR model (or the Gillespe-Nyholm rules)
The premise of the VSEPR model is simplicity itself: clumps of electrons repel each other. Thus, the atoms in a molecule will be arranged so that the electron clumps get as far apart as possible, as this minimizes the repulsions between the electrons. This model requires the shift in thinking mentioned above. When we ask about the shape of a molecule, we are asking about the arrangement of the atoms in space; but the position of the atoms is determined by the arrangement of the electrons. You must first think about where the electrons are, and then, and only then, think about the atoms. (Remember THINK ELECTRONS)
Note: the VSEPR model says nothing about orbitals the electrons sit in vaguely-defined clumps. We need not worry about all that orbital-business for this model. All we need to do is count the electrons, and then get electron clumps as far apart from each other as possible. (Not to worry the orbitals will appear in the VBT model.) The term “electron clump” is very useful when dealing with multiple bonds.
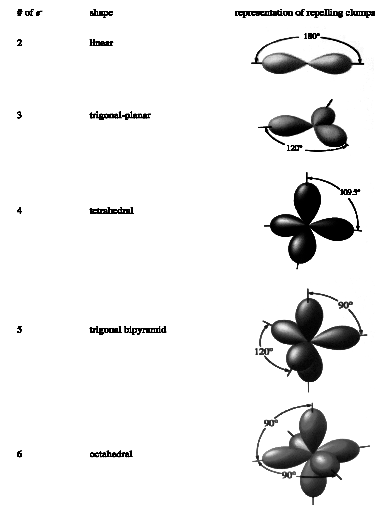
With such a simple premise -- clumps of electrons repel each other -- our model is also quite simple, once we know how many clumps of electrons are around the central atom. Consider the shapes that result when various numbers of electron clumps surround a given element. Remember: these shapes are determined by the clumps getting as far from each other as possible.
To predict the shape of a simple molecule using the VSEPR model:
i) draw a correct Lewis diagram,
ii) determine the number of e clumps around the central element,
iii) assign the correct shape predicted by that number of electron pairs,
iv) attach the necessary nuclei to the bonding electron pairs.
Figure 1: VSEPR Model Representations
Valence Bond Theory (VBT) and Hybridization
A basic postulate of most bonding models (including VBT) is that a covalent bond forms between two atoms when those atoms share at least one pair of electrons. The assumption here is that when electrons are shared, that means that they are sitting in the region of space between the two nuclei; to emphasize this crucial idea one more time - bonding occurs when an electron pair resides between two atoms. This can happen when two atomic orbitals overlap in this interatomic region and when there is an electron pair to go into the overlap region.
For example, consider a molecule of hydrofluoric acid. The valence electron of the H atom resides in the spherical 1s orbital, and the odd valence electron of the F atom is in a 2p orbital. The 2p orbital is not spherical, but has a node through the nucleus, and is shaped somewhat like a dumbbell. When these two atomic orbitals overlap and share the two electrons, a sigma bond results (Figure 2).
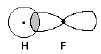
Figure 2: Valence Bond Theory Representation of HF
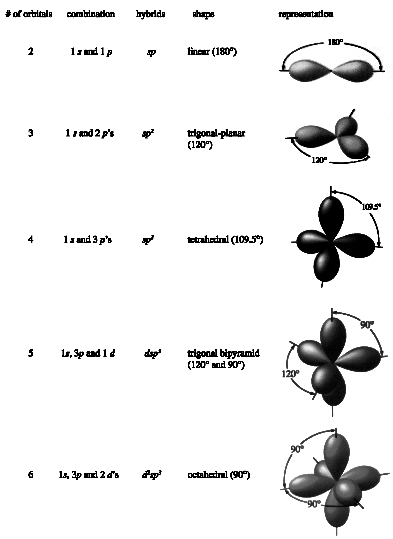
Linus Pauling proposed that the shape of a molecule (or a portion of a molecule) is determined by the orientation of the atomic orbitals around the central atom. Pauling noted this problem and proposed that these "native" atomic orbitals (the s, p and d orbitals) are not used in such molecules. In their place, "hybridized" orbitals are formed from combinations of the native s, p and d orbitals, and it is the shapes of these hybridized orbitals which determines the shape of the molecule. As discussed in your text, there are many possible ways to combine the s, p and d orbitals, and a variety of shapes result; the most important ways are shown below.
Other combinations of s, p and d orbitals are possible, but these cover all of the cases we will be seeing this year, as they cover all but a very few (and very strange) molecules. Note: the shapes predicted by the two models (VSEPR and VBT) are identical.
To summarize, according to these models, the shape of a molecule is determined by:
VSEPR - the number of electron clumps around the central element
VBT - the hybridization of the orbitals involved in the bonding.
Polarity
Once the three-dimensional structure of a molecule is determined, its polarity can be estimated. To do this, two factors must be considered: the polarity of the individual bonds, and the shape of the molecule.
If a bond forms between two atoms of different electronegativities, the bond will be polar. The more electronegative atom of the bonding pair will have a larger share of the bonding electrons, and will be negatively charged with respect to the less electronegative atom. The greater the difference in their electronegativities, the more polar the bond, and the greater the charge separation between the less and more electronegative atoms. The magnitude of the charge separation is called the bond dipole, and is generally represented by an arrow (actually a vector), with the negative (electron-rich) end at the head of the arrow, and the positive, electron-deficient, end indicated by the + sign at the tail of the arrow. For example, both HF and HCl are polar molecules, as the H is less electronegative than either F or Cl, but since F is more electronegative than Cl, the HF molecule is more polar than HCl.
![]()
H-F has a larger bond dipole than H-Cl (this is denoted by the longer dipole arrow over the H-F bond).
For molecules with more than one bond, the polarity of the entire molecule depends on its three-dimensional structure. Consider BF3 and NF3. The BF3 molecule is planar with the 3 F atoms at the corners of an equilateral triangle, with F-B-F angles of 120”. Us. Using the VBT, one would say that the geometry of BF3 is due to the sp2 hybridization of the B orbitals, and we reach the same conclusion about the molecular geometry. Because BF3 is triangular planar, the individual bond dipoles cancel, and the molecule is nonpolar.

On the other hand, a molecule of NF3 has two more electrons than BF3 , the atoms in NF3 form a triangular pyramid, with a lone pair of electrons in the fourth orbital (an sp3 hybrid orbital in VBT) above the nitrogen atom. The individual dipole moments do not cancel in NF3, so the molecule is polar. We say that NF3 has a dipole moment, or that it is polar.
The Experiment:
![]() Students will work in pairs for this experiment (actually a lab exercise). The laboratory report sheets for experiment 9-016 are due at the end of the lab period this week.
Students will work in pairs for this experiment (actually a lab exercise). The laboratory report sheets for experiment 9-016 are due at the end of the lab period this week.
Using your molecular model kit, construct models of the molecules or ions on one of the lists assigned to you by the laboratory instructor.
For each molecule or ion, record the following information in your laboratory notebook:
• Draw a Lewis structure and indicate the number of bonding and non-bonding clumps of electrons around the central element.
• Draw a sketch of the molecular shape predicted by VSEPR, indicating the positions of atomic centers and nonbonding electron pairs.
• Using VBT, identify the hybrid orbitals used by the central atom.
• For the neutral molecules in your set, predict the magnitude and direction of the dipole moment, if any.