

Experiment 3-080 Spectrophotometric determination of phosphate in a soft drink
Report Sheet for experiment 3-080: Extra report sheet (pdf format).
![]() In
this experiment you will determine the phosphate content of a
commercially available soft drink. Phosphoric acid (H3PO4)
and its salts are important components of biological fluids and
fertilizers. Phosphates are added to soft drinks as a flavor
ingredient and as an evanescent (they aid the fizziness). Since
phosphoric acid (H3PO4) and its anions (H2PO4,
HPO42-, and PO43-) are
colorless, they cannot be directly determined using visible-light
spectrophotometry. Instead, we will quantitatively convert them
into a colored substance, whose absorbance can be easily
measured.
In
this experiment you will determine the phosphate content of a
commercially available soft drink. Phosphoric acid (H3PO4)
and its salts are important components of biological fluids and
fertilizers. Phosphates are added to soft drinks as a flavor
ingredient and as an evanescent (they aid the fizziness). Since
phosphoric acid (H3PO4) and its anions (H2PO4,
HPO42-, and PO43-) are
colorless, they cannot be directly determined using visible-light
spectrophotometry. Instead, we will quantitatively convert them
into a colored substance, whose absorbance can be easily
measured.
![]() To
do this, we will react the phosphates in the soft drink with the
molybdate ion, MoO32-. The initial product
of this reaction is the phosphomolybdate ion, [PO4.•12MoO3]3-.
This complicated monster is also colorless, but when reduced
(we'll use SnCl2) it turns into a material, of unknown
composition, called molybdenum blue. Molybdenum blue is
intensely colored (guess what color), and when we measure the
concentration of this material, we can relate that concentration
to the concentration of the phosphates present in the initial
soda.
To
do this, we will react the phosphates in the soft drink with the
molybdate ion, MoO32-. The initial product
of this reaction is the phosphomolybdate ion, [PO4.•12MoO3]3-.
This complicated monster is also colorless, but when reduced
(we'll use SnCl2) it turns into a material, of unknown
composition, called molybdenum blue. Molybdenum blue is
intensely colored (guess what color), and when we measure the
concentration of this material, we can relate that concentration
to the concentration of the phosphates present in the initial
soda.
![]() Students will need to prepare a standard series of solutions and
individual samples of a chosen soft drink. Students should work
with a partner to prepare the standard series and then work
independently to prepare separate soft drink samples.
Students will need to prepare a standard series of solutions and
individual samples of a chosen soft drink. Students should work
with a partner to prepare the standard series and then work
independently to prepare separate soft drink samples.
Students will use volumetric glassware to prepare their solutions for analysis. Analysis will involve the use of spectroscopy. There is a section on Spectrophotometers and Spectroscopy in the laboratory manual (p. 43). Students should read this section carefully before coming to lab.
The standard series will be used to plot a standard curve of Absorbance vs Concentration of Phosphate (ppm):
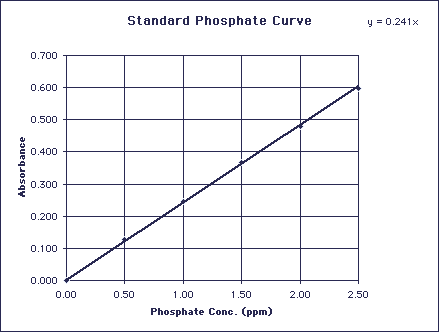
Figure 1: Standard Absorbance Curve of Phosphate at 690 nm
Once a standard absorbance curve has been generated, one can find the concentration of phosphate in a soft drink sample by employing Beer's law:
A =elc
where A= absorbance, e = absorptivity coefficient, l = path length and c= concentration
Beer's Law gives us an equation of a straight line (y = mx + b) with the y-intercept (b) = 0. In this experiment our standard curve is a straight line (or should be), so we can equate slope of the standard curve with el. We will use 1.00 cm path length cuvettes for the spectrophotometric analysis of our phosphate standard soltuions and soft drink samples (thus l = 1.00 cm) and so e will simply equal the slope of the standard curve. We will measure the absorbance (A) of the soft drink samples and then solve for the concentration (c) of phosphates in the soft drink.
Graphing Using Excel: Instructions using Excel to graph your data.
Dr. J's White Board Notes (PDF format)