
Lab 10 report sheets are due this week. Lab 11 report sheets will be due the week of December 7, 2009

Check-Out is scheduled for the week of December 7, 2009. You must check-out of lab or you will be charged a $50.00 check-out fee.
Experiment 11-034: Analysis of a ZN/Al Mixture
Report Sheets for Experiment 11-034: pdf format
This week you are going to determine the composition of a mixture of metals (Al and Zn) by measuring the amount of hydrogen gas released when the metals react with hydrochloric acid. A an alloy of Zn and Al will be reacted with the acid, and by measuring the initial mass of the mixture used, and the amount of H2(g) released, the ratio of Zn to Al in the mixture can be determined.
Some metals often called the active metals react in acid solution to produce hydrogen gas and a cation of the metal:
Zn(s) + 2 H+(aq) ---> H2(g) + Zn2+(aq)
From the stoichiometry of this balanced reaction, it is clear that 1.00 mole of solid zinc could produce 1.00 mole of H2(g). So, if the amount of H2(g) released in this reaction is measured, then the amount of zinc which was used to produce it could be determined. While the stoichiometry differs when aluminum reacts:
2 Al(s) + 6 H+(aq) -----> 3 H2(g) + 2 Al3+(aq)
the amount of H2(g) released can still be used to determine the amount of aluminum metal reacted. The stoichiometries of the above reactions differ, as do the atomic weights of zinc and aluminum, so equivalent molar amounts of these two metals will release different amounts of hydrogen gas.
![]() Experimentally, a massed sample of an alloy of Al and Zn will be completely reacted with HCl, and the resulting H2(g) will displace a measurable volume of water. The apparatus for such a study is Florence flask.
Experimentally, a massed sample of an alloy of Al and Zn will be completely reacted with HCl, and the resulting H2(g) will displace a measurable volume of water. The apparatus for such a study is Florence flask.
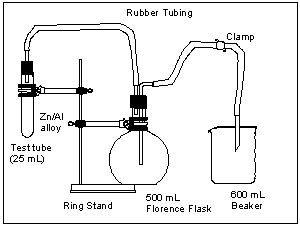
Figure 1. The Florence flask apparatues
When the HCl is added to the sample in the test tube, H2(g) is released, displacing water in the Florence flask, which is collected in the beaker. To determine the moles (n) of H2(g) released, a rearranged form of the Ideal Gas Law can be used:
n = PV/RT
This equation shows that n (the moles of H2(g) released) can be determined if the pressure (P), volume (V) and temperature (T) of the hydrogen gas are known. The temperature of the H2(g) is the temperature of the room, the volume of the H2(g) is the volume of the water it displaces. The experiment can be designed so that the pressure of the gas trapped in the Florence flask is the same as the atmospheric pressure in the room, which can be measured with the barometer in the laboratory.
The only semi-tricky bit here is that the gas trapped in the Florence flask consists of the H2(g) released by the metals, as well as water vapor from the water, which is not displaced, in the flask. If the height of the beaker is adjusted so that the top surface of the water in the beaker is at the same level as the water left in the Florence Flask (Figure 2) then the pressure in the Florence flask (Ptotal) is the same as atmospheric pressure (Patm).
But since the gas in the Florence flask consists of H2(g) and H2O(g), Dalton's Law of Partial Pressures can be used:
Ptotal = PH2 + PH2O = Patm
Rearrangement gives:
PH2 = Patm PH2O
Remember - Patm can be measured using the barometer in the lab, and, since the vapor pressure of water (PH2O) depends only on the temperature (and can be found in standard tables), the partial pressure of hydrogen (PH2) is readily calculated.
Table 1: Vapor Pressure and Density of Water (17 oC - 28 oC)
| T (°C) | Density (g/mL) | T (C) | Density (g/mL) | T (C) | Density (g/mL) |
|---|---|---|---|---|---|
| 17.0 | 0.99877 | 21.0 | 0.99799 | 25.0 | 0.99704 |
| 18.0 | 0.99860 | 22.0 | 0.99777 | 26.0 | 0.99678 |
| 19.0 | 0.99841 | 23.0 | 0.99754 | 27.0 | 0.99651 |
| 20.0 | 0.99820 | 24.0 | 0.99730 | 28.0 | 0.99623 |
| Vapor Pressure(mm Hg)
T (°C) |
0.0 | 0.2 | 0.4 | 0.6 | 0.8 |
| 17 | 14.530 | 14.715 | 14.903 | 15.092 | 15.284 |
| 18 | 15.477 | 15.673 | 15.971 | 16.071 | 16.272 |
| 19 | 16.477 | 16.685 | 16.894 | 17.105 | 17.319 |
| 20 | 17.535 | 17.753 | 17.974 | 18.197 | 18.422 |
| 21 | 18.650 | 18.880 | 19.113 | 19.349 | 19.587 |
| 22 | 19.827 | 20.070 | 20.316 | 20.565 | 20.815 |
| 23 | 21.068 | 21.324 | 21.583 | 21.845 | 22.110 |
| 24 | 22.377 | 22.648 | 22.922 | 23.198 | 23.476 |
| 25 | 23.756 | 24.039 | 24.326 | 24.617 | 24.912 |
| 26 | 25.209 | 25.509 | 25.812 | 26.117 | 26.426 |
| 27 | 26.739 | 27.055 | 27.374 | 27.696 | 28.021 |
| 28 | 28.349 | 28.680 | 29.015 | 29.354 | 29.697 |
CALCULATIONS:
If our sample had been pure Zn, we could say: n of H2(g) = nH2 = nZn
If our sample had been pure Al, we could say: n of H2(g) = nH2 = (3/2) nAl
Since we are dealing with a mixture of the two, we can write:
nH2 = nZn + (3/2) nAl
We also know that the mass of our sample (gtotal ) is the mass of the Al plus the mass of the Zn:
gtotal = gzn + gAl
or by rearrangement:
gzn = gtotal - gAl
Since: moles = mass(g)/Atomic Mass , we can rewritethe above equation as:
nH2 = [gzn/65.4 g/mole] + [(3/2)[gAl/27.0 g/mole]
Now substituting,, we get:
nH2 = [(gtotal - gAl)/65.4 g/mole] + (3/2)[gAl/27.0 g/mole]
or with some rearrangement,
nH2 = 0.0153(gtotal) - 0.0153(gAl) + 0.0556(gAl)
and more simply,
nH2 - 0.0153(gtotal)= 0.0403(gAl)
So... we now have an equation with three variables:
But you know 1 and 2 from your experimental results. So all you need to do is solve the last equation for gAl, plug in the values for the moles of hydrogen generated and the grams of metal initially used, and you can solve this equation for the g of Al in your sample. Once you know the grams of Al in your sample, can the g of Zn and the % composition be far behind?