 |
|
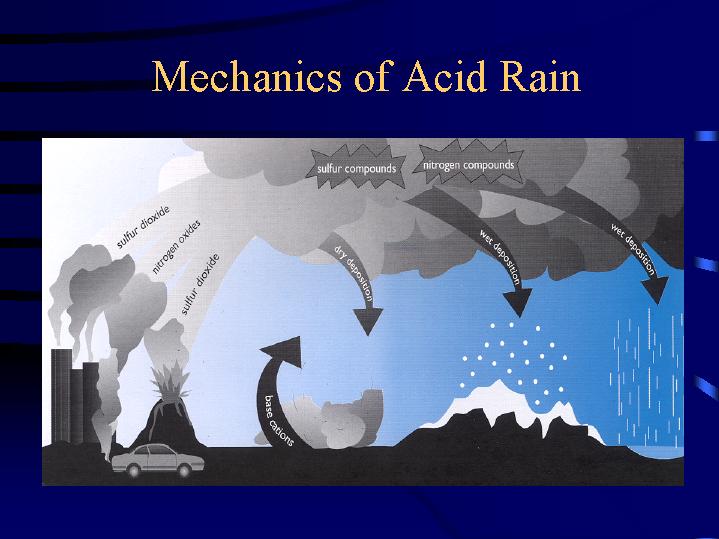
Scientists have discovered that air pollution from the burning of fossil fuels is the major
cause of acid rain. Acidic deposition, or acid rain as it is commonly known, occurs when
emissions of sulfur dioxide (SO2) and oxides of nitrogen (NOx) react in the atmosphere with
water, oxygen, and oxidants to form various acidic compounds. This mixture forms a mild solution
of sulfuric acid and nitric acid. Sunlight increases the rate of most of these reactions. Acid rain causing pollution is carried on prevailing winds and can drift for hundreds of miles before it is deposited by precipitation. Adirondack, Catskill and Appalachian mountain regions are the hardest hit because prevailing winds carry the pollution from several other states onto those mountain ranges (which we will see in a few slides). As the winds rise over the mountains, the moisture they contain cools and condenses into the clouds, which reach the point of saturation. The resulting "rain" has high concentrations of sulfur and nitrogen pollution. The sulfur dioxide becomes sulfuric acid, and nitrogen becomes nitric acid. Rich April revealed the statistic that in the Adirondacks only 20 percent of the acid rain has a natural source.
| |
|